Diamonds will help to safely dispose of synthetic dyes
22 June 2020 г.
Aromatic compounds are widely used in the textile, paper, leather industry, and production of medicines and cosmetics, as well as food. These substances and their decomposition products contained in wastewater are toxic and have a detrimental effect on human health and natural ecosystems. Moreover, such chemicals are difficult to oxidize, that is, they are difficult to destroy by the usual biological, physical or chemical methods. Researchers are developing innovative electrochemical technologies, which are based on the use of strong oxidizing reagents that can significantly increase the efficiency of the hazardous substances destruction.
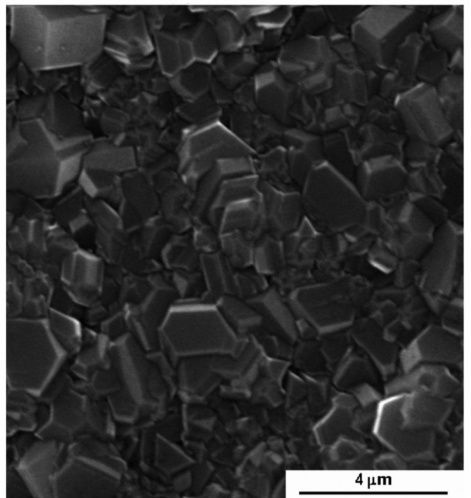
Scientists at the Federal Research Center “Krasnoyarsk Science Center SB RAS” identified the optimum conditions for the destruction of such pollutants as aromatic substances. They proposed to oxidize toxic biodegradable organic compounds by the electrocatalytic method using a diamond electrode with the addition of boron to increase the electrical conductivity of the material.
To develop an effective method for disposing of aromatic pollutants, the researchers studied how the electrocatalytic oxidation would proceed using various types of materials for the anode. The electrode material was found to significantly affect the efficiency of electrochemical decomposition of substances. The scientists attribute this to the fact that, depending on the type of the electrode material, additional oxidizing particles, namely hydroxyl radicals and active oxygen, with different intensities and efficiencies are formed on the electrode, increasing the rate of oxidation and enhancing the destruction of pollutants. With a high content of such radicals, toxic substances can completely "turn" into water, carbon dioxide and minerals.
Electrodes with different electrocatalytic activity were tested. The largest number of oxidizing reagents is formed on a diamond electrode doped with boron, while the smallest - on ruthenium and titanium dioxide. The high oxidizing ability of the diamond structure is associated with a very weak interaction of oxidizing particles with the surface of the electrode, which contributes to their rapid reaction with an organic substrate.
“Anodic oxidation technologies are increasingly considered as alternative methods for treating industrial wastewater containing various organic and inorganic pollutants. In our case, for the destruction of aromatic compounds, the most effective of all the tested anodes is a diamond electrode doped with boron. It is used as a material for the oxidation of both organic and inorganic substances. With its help, it is possible to better and faster eliminate such persistent environmental pollutants as synthetic dyes, aniline, phenol, benzene and their derivatives, pesticides and herbicides, ” says Tatyana Kenova, Candidate of Chemical Sciences, senior researcher at the Institute of Chemistry and Chemical Technology FRC KSC SB RAS.
Share:
