Janus nanostructures could help produce green hydrogen
27 February 2023 г. FRC KSC SB RAS
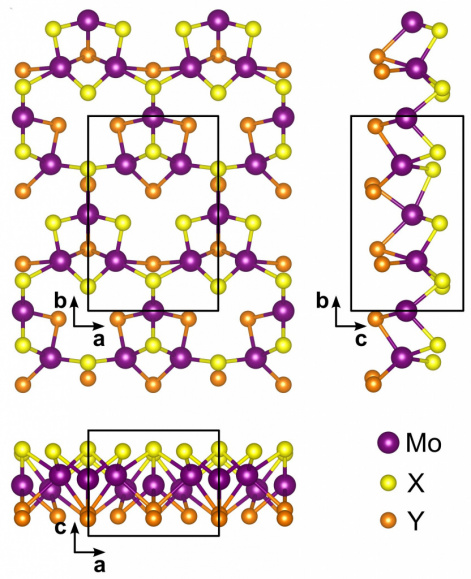
As early as in the 1970s, Japanese scientists were conducting research on the process of splitting water under the influence of sunlight. At that time, to accelerate the reaction, use was made of semiconductor catalysts based on titanium dioxide, but they showed low efficiency. Catalysts based on transition metal dichalcogenides turned out to be much more interesting because of their stability and diversity of structures and chemical compositions. Monolayers with a Janus nanostructure proved to be especially in demand for water splitting. They have this name because the upper and lower rows of atoms in these structures consist of different elements, meaning that they are two-faced, like a god from ancient Roman mythology. This feature of Janus monolayers makes it possible to accelerate the reaction of water decomposition under exposure to light.
A team of researchers including scientists from the Krasnoyarsk Science Center of SB RAS investigated a novel catalyst that uses the properties of Janus structures to produce hydrogen from water. The scientists took compounds based on sulfur, molybdenum, selenium and tellurium to conduct computer simulations of photocatalytic reactions in the presence of monolayer crystalline semiconductors with the Janus structure. The most promising candidate for the production of solar hydrogen proved to be a material based on the SMoTe compound (where S is sulfur, Mo is molybdenum, and Te is tellurium). The anticipated solar-to-hydrogen conversion efficiency was 54% and 67.1% for neutral and acidic environments, respectively, which is well above the generally accepted commercialization limit of 18%. Other compounds (SMoSe, SMoO, SeMoO and SeMoTe, with O being oxygen), used by the researchers in theoretical calculations, are also suitable for hydrogen extraction from water with an efficiency above the specified limit, and theses can be used in different environments (either in neutral or acidic conditions, depending on the chemical composition of the compound).
Though the research is based on the calculated data, the results can be used in practice. Today, this is especially relevant, since developments in the field of green energy are popular, and in the future they can at least partially replace the traditional approaches in terms of fossil energy sources and thereby reduce the carbon footprint of mankind. Industrial companies are already showing interest in greener renewable fuels, and their application is possible everywhere in the world, and not just in regions with a lot of sunny days per year, as the researchers say.
“Sunlight-assisted water splitting is of practical interest because using the resulting hydrogen could reduce greenhouse gas emissions, meet growing global energy demand, and address the challenges of sustainable energy supply around the world. In our work, we have shown that the family of catalysts studied contains new dynamically stable structures with outstanding properties for practical application,” says project supervisor Zakhar Popov, Candidate of Physical and Mathematical Sciences, senior researcher at the Institute of Biochemical Physics of RAS.
“At the initial stage of the study, using the predicted structural parameters and elemental composition of new semiconducting materials, we simulated their properties. The next step was synthesis. In order to perform quality control of the obtained nanostructures, for example, to determine the number of layers or to monitor possible defects, one can use Raman spectroscopy. In our work, we show in advance what spectra would be obtained. Thus, we cannot only say which compositions are the most promising, but we can also provide information necessary for the structural characterization of future photocatalysts, ” comments a co-author of the article Alexander Oreshonkov, Candidate of Physical and Mathematical Sciences, researcher at the Laboratory of Molecular Spectroscopy of the L.V. Kirensky Institute of Physics KSC SB RAS.
The research was conducted by researchers from the N. M. Emanuel Institute of Biochemical Physics of RAS (Moscow), the Institute of Geology and Mineralogy of SB RAS (Novosibirsk), the L. V. Kirensky Institute of Physics of SB RAS (Krasnoyarsk) and the Siberian Federal University (Krasnoyarsk) and Novosibirsk State University (Novosibirsk).
The study was supported by the Russian Science Foundation.
Share:
